TABLE OF THE SPECIFIC HEAT OF GASES.
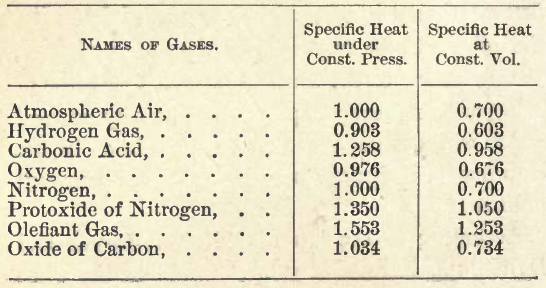
From this and others like it, they arrived at this ratio:
Gamma = Cp/Cv
Where Cp is the
"Specific Heat of gas under constant pressure"
and
Cv is the
"Specific Heat of gas under constant volume"
and Gamma is a very fundamental constant used in thermodynamics of gases.
But wait, we now know that gases always have the same specific heat. So how did they derive a real measurement of a real property of gases, from a non-existent difference in specific heats of gases?
|
How Very Quaint(er)!
A second conundrum faced scientists in the early 1800's. One of the theories that was explored was the Specific Heat of Volume.
This seems absurd when looked at with the benefit of Ideal gas Law, general understanding of specific heat and Laws of Thermodynamics. So is it because it takes a bigger fireplace to heat a bigger room?
Quite the contrary, the idea of multiple "Specific Heats" came up a result of very precise measurements. In that era, scientists were cataloging material properties for different compounds. Specific heat being one of them. Measured with great accuracy and repeatability, until they got to gases.
Consider how one would measure specific heat of a gas. One has to enclose it, and account for the specific heat of the enclosure. Then find an accurate way of adding a measured amount of heat, with flame? Heated electrical element? Hot water? And then they measured that to 3 decimal places. So the problem is this, you get a beaker full of gas, put in a stopper so the air and gas don't mix, and heat it up exactly one degree. And the guy across the room has a sealed rigid chamber, and does the same thing. He gets a very different answer, 30% or more different. Why? The volume changed for the first measurement. How much? at room temperature 1/300th of the volume. If there were 100 millimeters of gas from bottom to top, it rose 1/300th of that, to 100.3 millimeters Easy to overlook.
They kept measuring and measuring, and eventually uncovered other properties of gas, like "Gamma" the exponent of a Work Pressure Curve.
Sorting this out was required to understand the relationship between Work and Heat.
|

