What is taught
It is taught diffusion is an inherently irreversible process.
A typical example, light a hot match in a room. The heat diffuses into the room, the area around the match which was hot will never be hot again.
However, as far as the thermodynamic behavior of the room, there is no difference between the room with a hot spot, or the room with evened out temperature The room contains exactly the same amount of energy in either case. So when it comes to vapors and engines, all that matters is the total heat.
Consider "real world engines, like Car engines, it makes no difference at all how the heat is introduced. Millions (maybe billions) have been spent on making the fuel air mixture evenly disbursed, and the net effect is either nothing, or negative. Why negative? because when fuel is burned in an open flame, it burns almost completely. The fuel-air combination is regulated by the combustion itself, and will tend to maximize combustion. If you mix fuel evenly with air, there may be insufficient concentrations for complete combustion. Result- carbon monoxide. So part of the fuel which could have pushed the car is uselessly burned in the catalytic converter. Waste of heat and more carbon in the air than necessary.
|
Reality, it depends
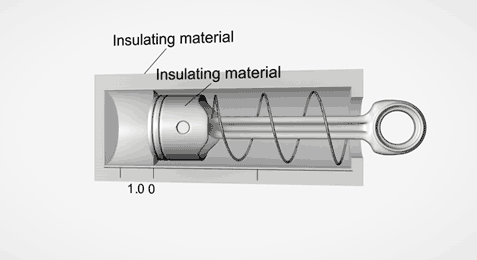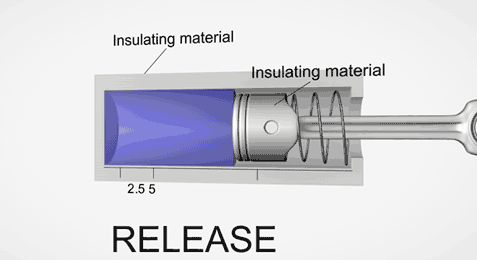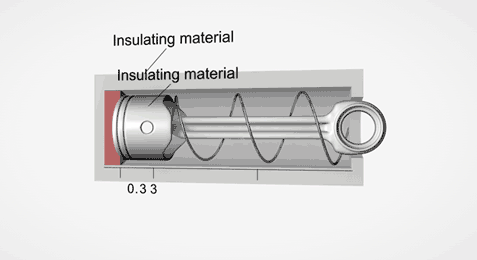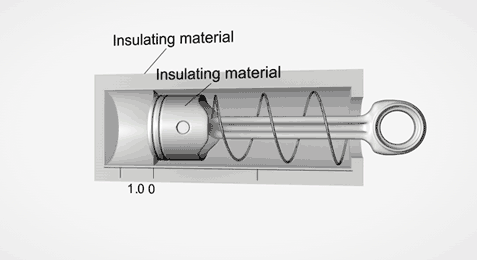 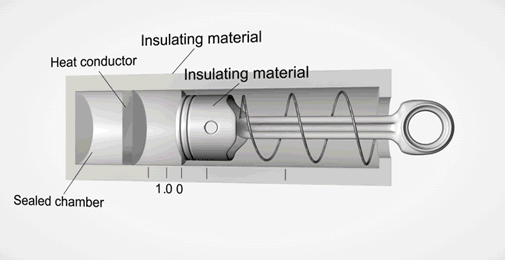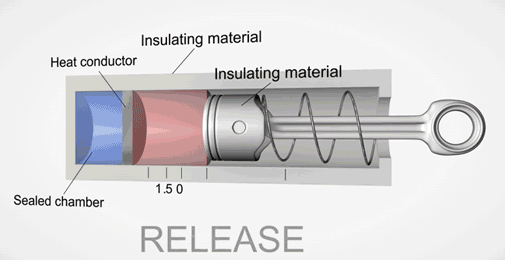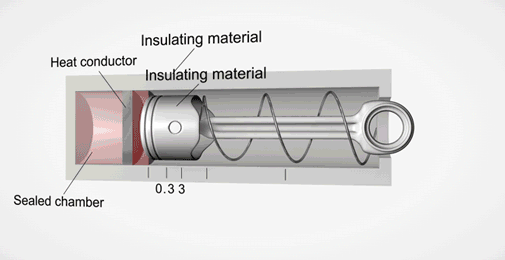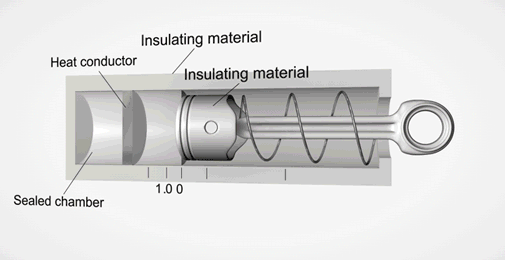
Take an ideal piston (on left - an insulated piston with perfect frictionless seal). It is a closed system. So if we displace it, using external work, it gets hotter or cooler in proportion. Allow the work to leave the system, temperature immediately returns to its beginning temperature (This is a review of work to heat transformation).
Now add diffusion (right). A second sealed chamber, but thermally coupled to the piston chamber. The chambers will adjust over time to equalize temperature with each other. Change the volume of the piston chamber, it immediately changes temperature Diffusion then equalizes temperature between chambers. Return the system to its initial state by allowing the total work done to the system to return to zero, the temperatures of both chambers must return to exactly their beginning temperature (Or the Law of Conservation of Energy is incorrect).
This is an example of strictly reversible diffusion.
|

