The myths- Impossible to build one.
- It is a uniquely best possible engine cycle, because it is the only "reversible" cycle.
- Since it is the only "reversible" cycle, it is the only cycle that it is a refrigeration cycle in reverse.
- Other engine cycles are less efficient at converting heat to work.
- The Carnot Ratio is the ratio of Heat converted to Work of the Carnot Cycle.
- The Work Ratio of other engine cycles is lower than the Carnot Cycle.
The Reality, a demonstration
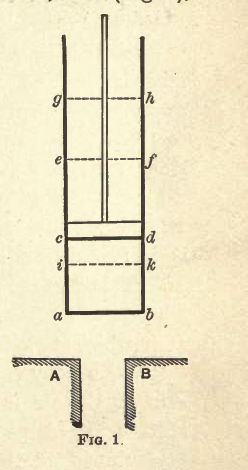 |
The Facts about a Carnot Cycle
- Sadi Carnot and Lord Kelvin both built one. Sadi Carnot's looks like the figure at left:
- Its a demonstration, in Sadi Carnot's words (he doesn't propose it be used as an engine cycle). In the early 1800's, the average classroom would have a wood burning stove. The device to the right and a wood burning stove can implement the famous Carnot Cycle. Ironically, today's classrooms cannot, because they don't have a wood burning stove.
Of course, FuelScience.Org isn't the final authority, so lets see what the Author, Sadi Carnot, said:
"Reflections" Pg 61.
We shall give here a second demonstration of
the fundamental proposition enunciated on page
56, and present this proposition under a more general
form than the one already given.
In multiple places Sadi Carnot refers to this cycle as a "Demonstration", and never refers to it as a superior cycle.
What is he demonstrating? "Reflections" Explains most heat engines of Sadi Carnot's era where Steam Engines. The primary thing the Carnot Cycle demonstrated was the feasibility of an air engine. Also, that an air engine would work as well as a steam engine. Attempts at air engines up until then appeared to be inherently less powerful than steam engines. Also demonstrated were Compression and Expansion using both the Adiabatic AKA Insulated, and Isothermal AKA Uninsulated methods. . Much of "Reflections" is devoted to steam engines. However, the fact that the type of vapor does not matter is stated multiple times. Use of air as the engine vapor has the obvious advantage that its available everywhere on Earth.
- All engine cycles are refrigeration cycles in reverse. In the "forward" direction, heat is added at the top (max pressure, min volume), and exhausted at the bottom (min pressure, max volume). A reverse or refrigeration cycle adds heat at the bottom (max volume, min pressure) and exhausts heat at the top (max pressure, min volume). The forward cycle produces work. The reverse cycle consumes work. For any cycle to be a refrigeration cycle, the heat added must come from the body to refrigerate, not from external fuel. The compression in a refrigeration cycle shifts temperature up, so it is capable of moving heat from one body to another, even if the destination body that heat is moved to is the same temperature or higher as the refrigerated area. The real proof here is you heard myth number three in an air conditioned room, which was not air conditioned by a Carnot Cycle.
- Heat is converted to and from work via Volume Change times Average Pressure. All engine cycles use this fact, and so are all equally good at the conversion, since there is only one shared conversion method. The same method applies to all Vapor Expansion engines (and refrigerators). The rate of conversion is 100%. Meaning whatever energy is converted in either direction, an exactly equal amount of energy shows up as converted. This is a necessary property of reversibility, and a necessary consequence of the Law of Conservation of Energy. Any other result would effectively allow creation or destruction of an amount of energy.
- The Work Ratio is not the ratio of the Carnot Cycle, but of any two points on the Work Transfer Curve. It can also be derived directly from the First Law of Thermodynamics, Conservation of Energy. More in following chapter. The Carnot Cycle converts a percentage of heat to work, and also loses a fixed percentage of heat by dissipating it to the atmosphere. The Work Ratio was never claimed as a unique solution of the Carnot Cycle by the author of either the cycle or the author of the ratio, but rather it applies to all engine cycles, because it is derived from the curve by which all engines convert heat to and from work.
- A typical automobile engine cycle is a 10 to 1 compression followed by a 1 to 10 expansion. Using the same geometry of volume change for a Carnot Cycle, and putting in the same amount of energy at the high pressure part of the cycle, the Carnot cycle would put the energy in spread out along the first about 1/2 of the expansion. The average expansion experienced by the energy added would be about 3 or 4. The automobile engine cycle adds all the energy at cycles beginning, so the energy goes through a complete 1 to 10 expansion,much more than the Carnot Cycle. Consequently, an automobile engine cycle has a Work Ratio aka Carnot Ratio computed from volume expansion of 10, or about 0.58, while the Carnot Cycle in the same cylinder with the same energy has a Work/Carnot Ratio of a 1 to 4 expansion, or about 0.40. To obtain the maximum Work Ratio, any engine cycle should put all the heat energy in at the very beginning of the cycle, before any expansion occurs.
|

