|
|
Is it too good to be true? Not quite.
So you are someone who has to read the fine print?
You know what that says about Trust Issues.
The previous pages engine can indeed work as advertised. However, it takes more than 2 volumes in general to return to near the original state (and having exported all the heat converted to work.) 2 Volumes would accumulate about .25 heat units per cycle as a result of recompressing. The 2 volume case can work in a vacuum, never exhaust heat, and exports all its work. However, part of the work exported is in lifting the piston weights incrementally each cycle, the machine grows. The other choices are outlined below. In a "pressure envelope" one choice is to use the envelope to reset without doing work, effectively abandoning some of the work as unexported. The spirit of the theoretical exercise is to demonstrate near 100% conversion and export of energy, which is discussed the next two pages in painful detail.
Work Inside the Engine
First, all the heat converted, was converted to work. There is no second choice.
After temperature exchange, there are three ways to continue to make an engine cycle (which returns within an arbitrarily small delta of its original state).
- If there is a pressure envelope, we can lock the piston before temperature switch, open the cylinder to the envelope, and restore it to any initial position we like, with effectively no work. The inside and outside temperatures are the same, so this would appear to release no heat.
|
Work Outside the Engine
The work outside the engine is exportable work. There was an excess of work created, allowing the air (pressure envelope) or the piston weights to be lifted. That work is exported already.
The rest of the work done, is stored in the weight or pressure envelope lift.
- The lifted weight or lifted pressure envelope, which is the same weight, fall. Effectively the potential energy is converted back to heat, the same way it would for any object dropped. The heat is dissipated. This method requires a pressure envelope, and also adds heat to the pressure envelope, by doing a net amount of work lifting it, and dropping it.
|
- If the piston is left free during the transfer of temperature to the other cylinder, the work done inside the piston is a pressure of "1" times distance. In other words, the same as was "stored" instead of exported during expansion.
|
- If the piston is left free during the transfer of temperature to the other cylinder, the work done inside the piston is a pressure of "1" times distance. In other words, the same as was "stored" instead of exported during expansion. The energy is then stored again as heat inside the cylinder. Since it happens during temperature switch, most of the added heat will go to the opposite cylinder. This method does not export any work, nor does it return the current piston all the way back to its original temperature It will have some heat increase and some volume increase.
|
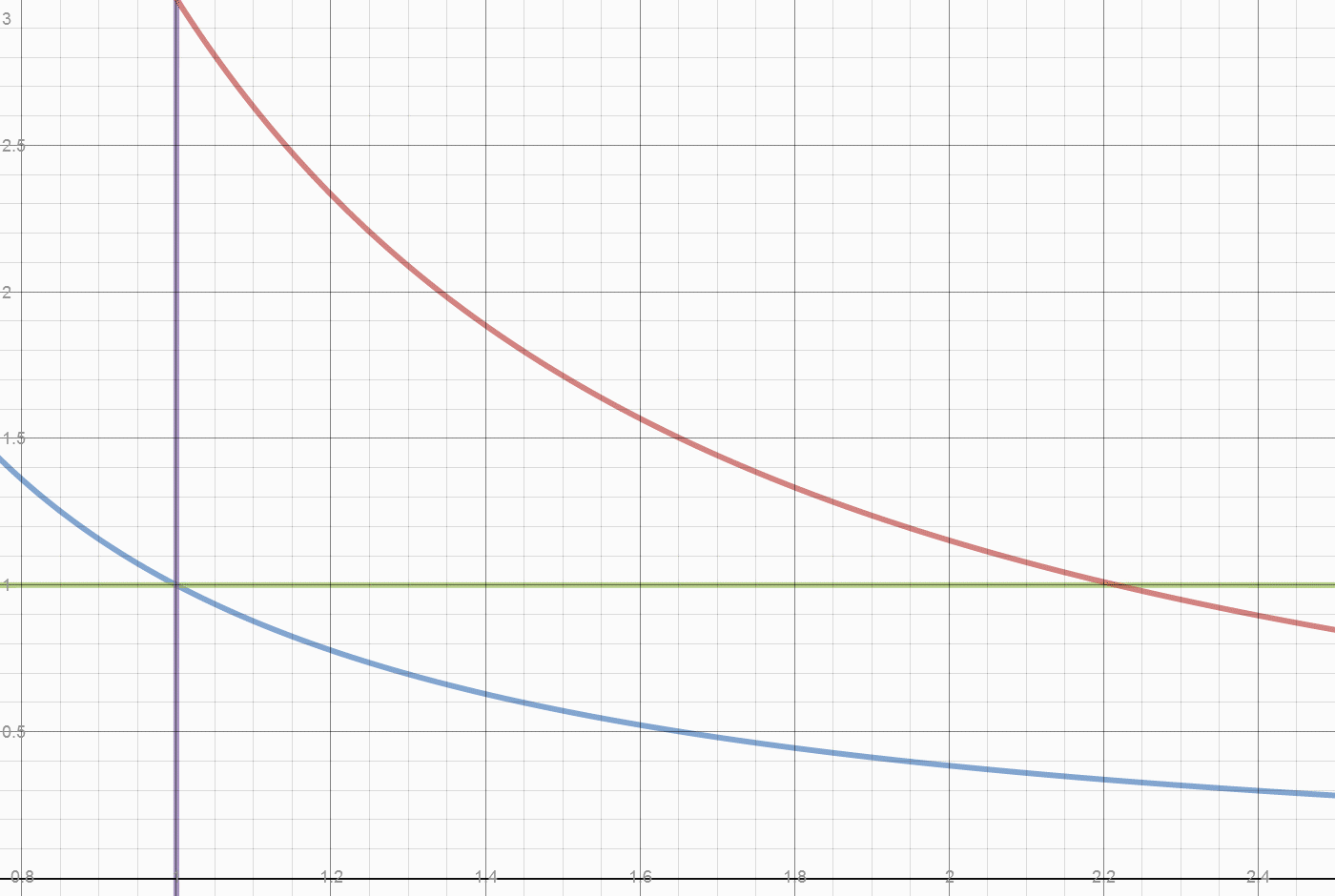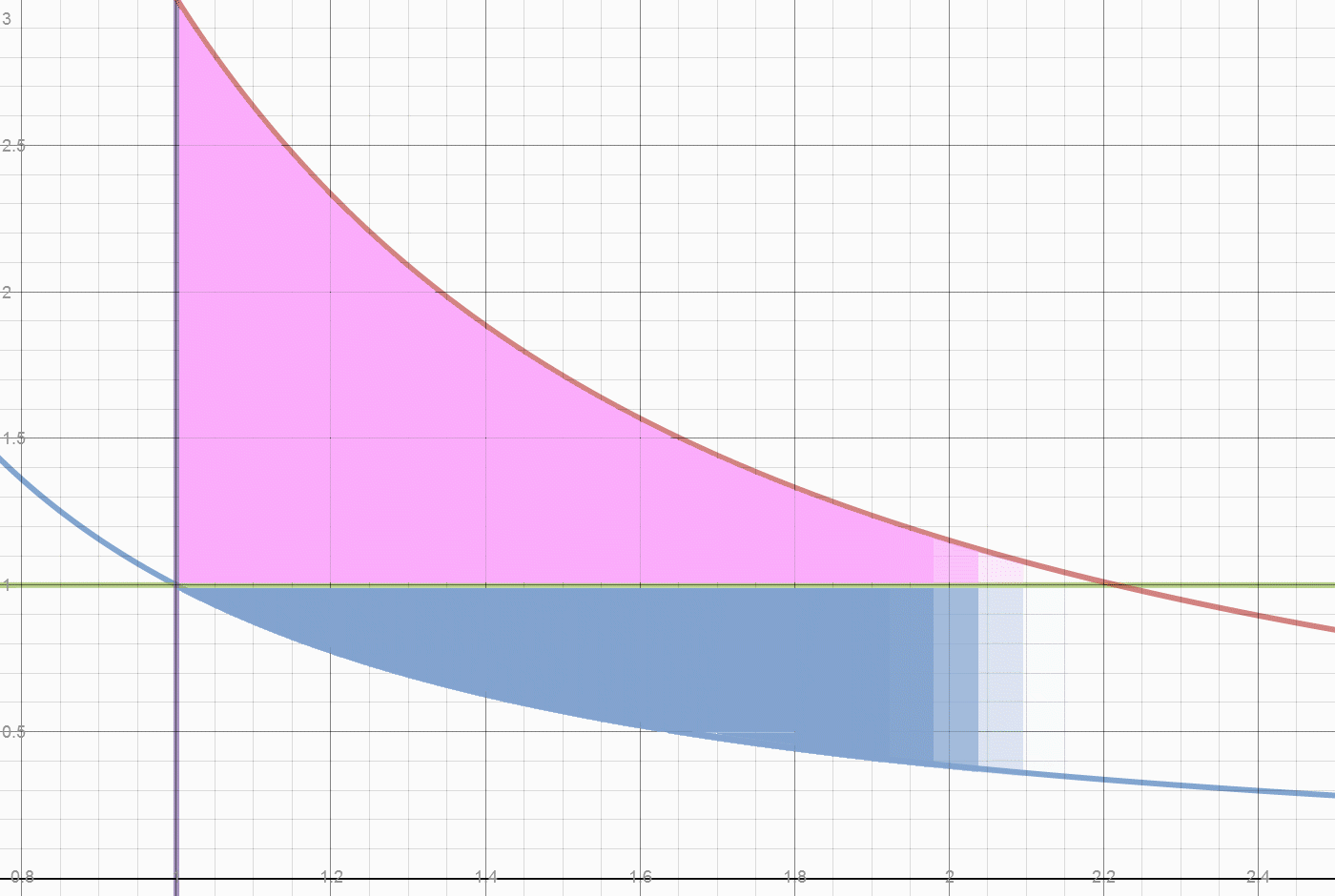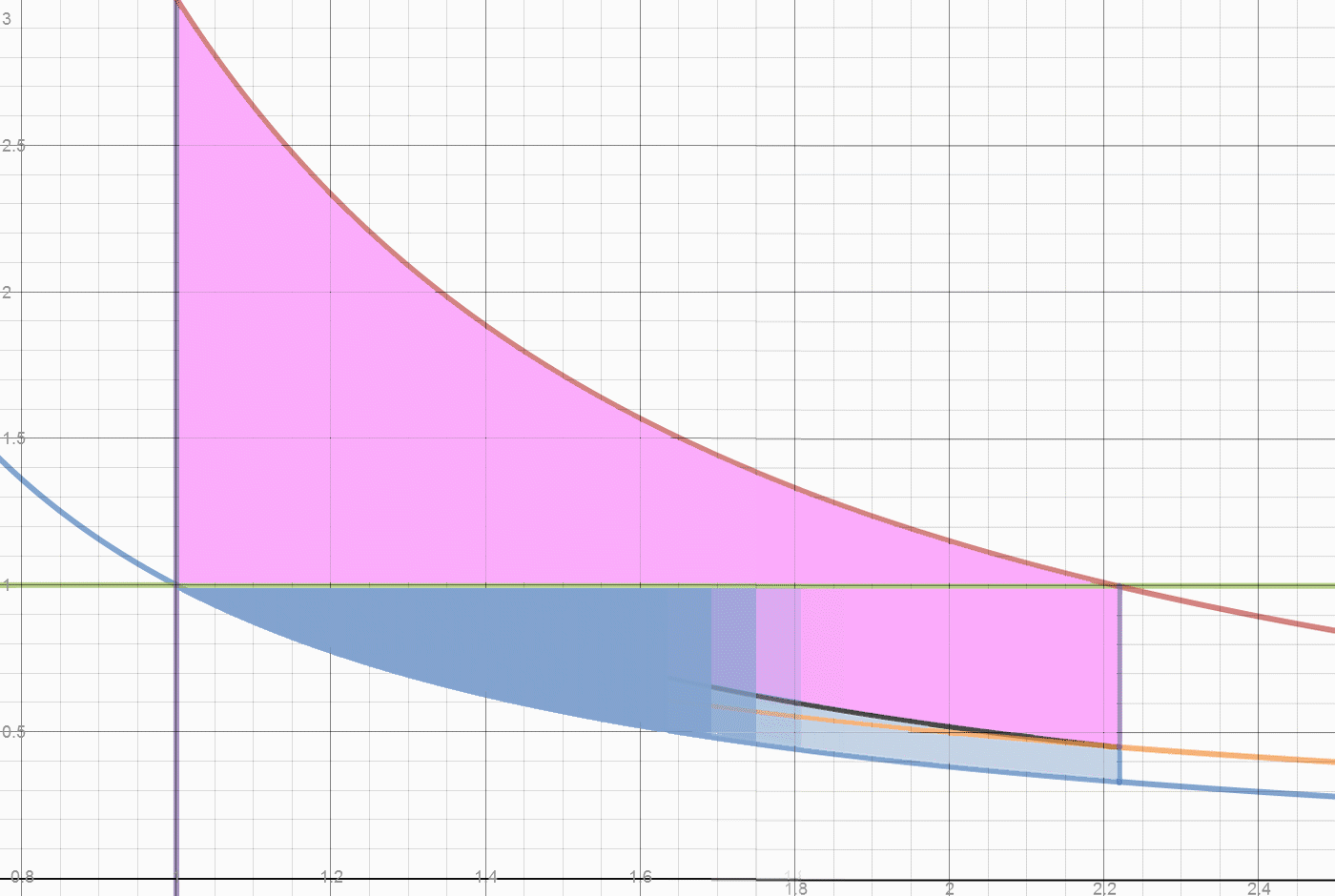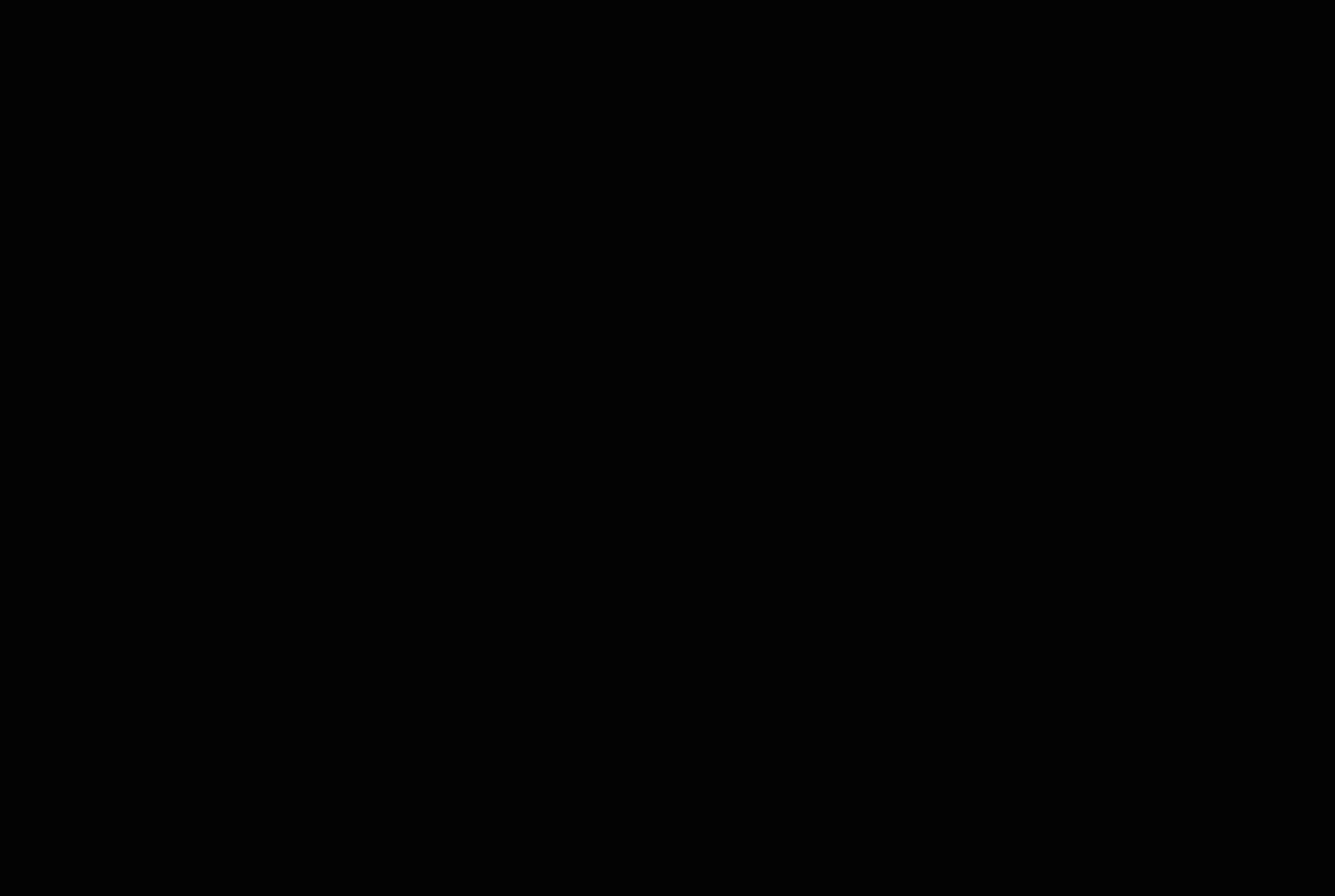
- If the piston is locked during the transfer of temperature to the other cylinder, the heat of air in the piston will return to its starting value, and the volume will remain 2.22 units. The pressure is then 0.45 times the pressure generated by the weight (or envelope). The work done inside the piston begins with a pressure difference of 0.65 pressure at position 2.22 and diminishes to 0 difference at position 1.25. This leaves 0.25 energy units stored in the weight lift, and the same (0.25 units) stored as heat.
|
- Initially 0.78 (2-1.22) of 2 units of heat were converted to work. They created 0.78/.4 =1.95 work area. Of that 1.22 units were used to lift the piston weight, leaving 0.73 work units exported. A return stroke exports work ABOVE the work curve, below the weight line. The total return work is 1.22 -0.25 = .97 total units. Subtract work below the curve of
0.97- (0.25/0.40)=0.97-0.62=0.35 exported work. Total exported work = 0.73+.35 =1.08 of 1.95 total units. There is still .25 heat units and 0.25 work units inside the engine. Total unexported work .87 work units or .35 heat units. That is what a two piston system exports. Note that all energy is accounted for, nothing is dissipated or lost.
1.08/1.95 = 55% converted and exported, right?
Wrong.
Herein lies what may be one origin of the "Carnot Limit" (apologies to Carnot, who claimed nothing of the sort.) Of the 0.78 temperature drop, only 2/3 came from the 2 units of heat added. A 100% conversion of the FUEL heat of 2 units, would leave the initial state of 1 unit of heat in each cylinder, and leave the initial density of 1 unit of vapor per 1 unit of volume. The Blue line "divides" the fuel heat from the initial energy. At any point on the blue line, releasing the weight simply returns the system to the initial state. This energy isn't convertible or exportable, it is the initial and final state of energy in the system. The Actual FUEL heat converted is 2/3*0.78 or 0.52 units. The work done by 0.52 units of heat is 0.52/0.4 or 1.3 units. We have exported 1.08/1.30 or 83% of the converted Fuel heat as useful work. Dropping the weight does the valuable work of restoring compression to the system, which requires work to be done on the contained vapor.
|
The next diagram shows how heat is divided between initial heat and fuel heat. The initial system heat is not accessible for export. The heat accessible for export creates a pressure difference relative to the initial state.
Recall the engine runs in a vacuum. The initial state is a weight placed on a piston. That weight determines the internal pressure of the system. The system also has an initial internal temperature.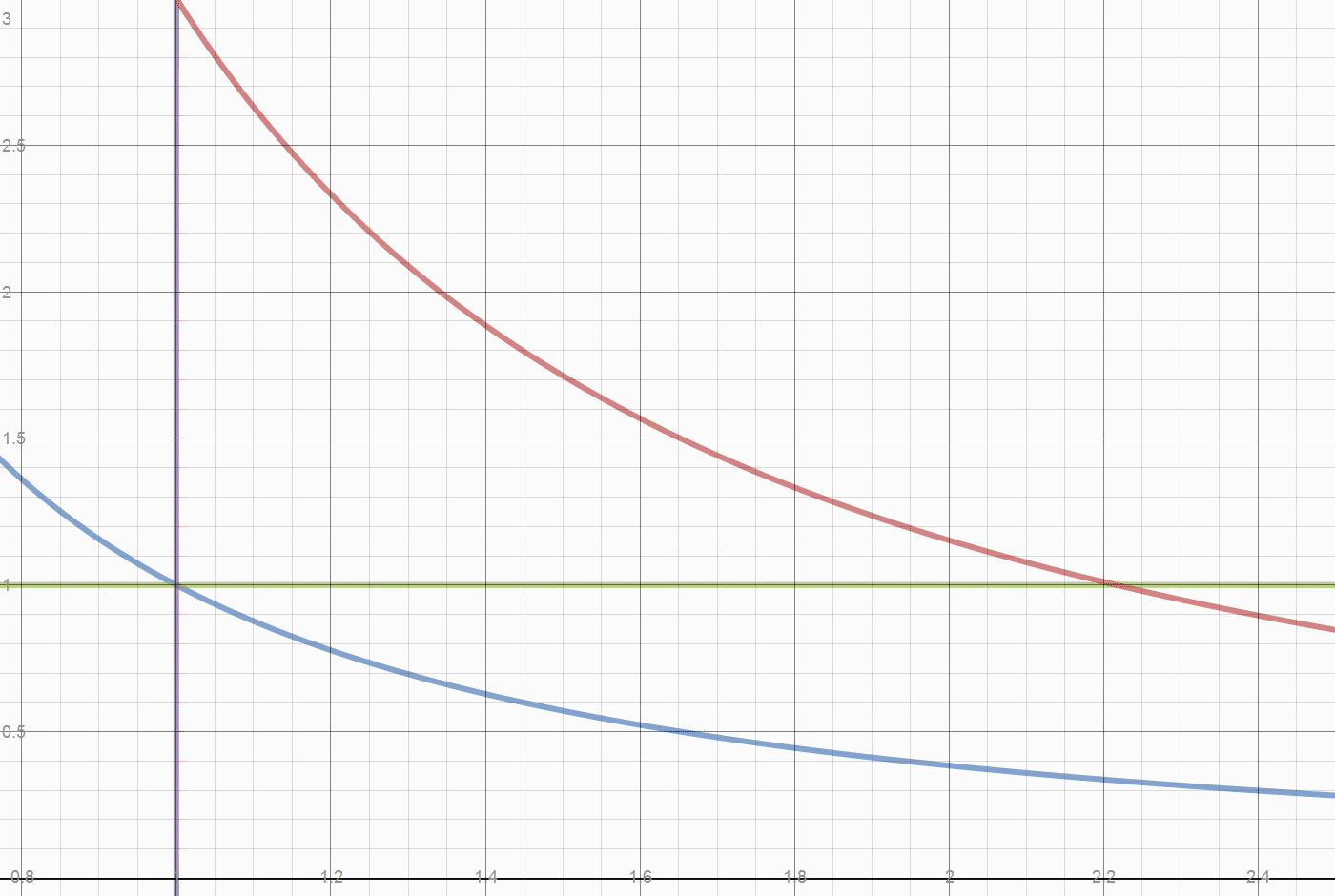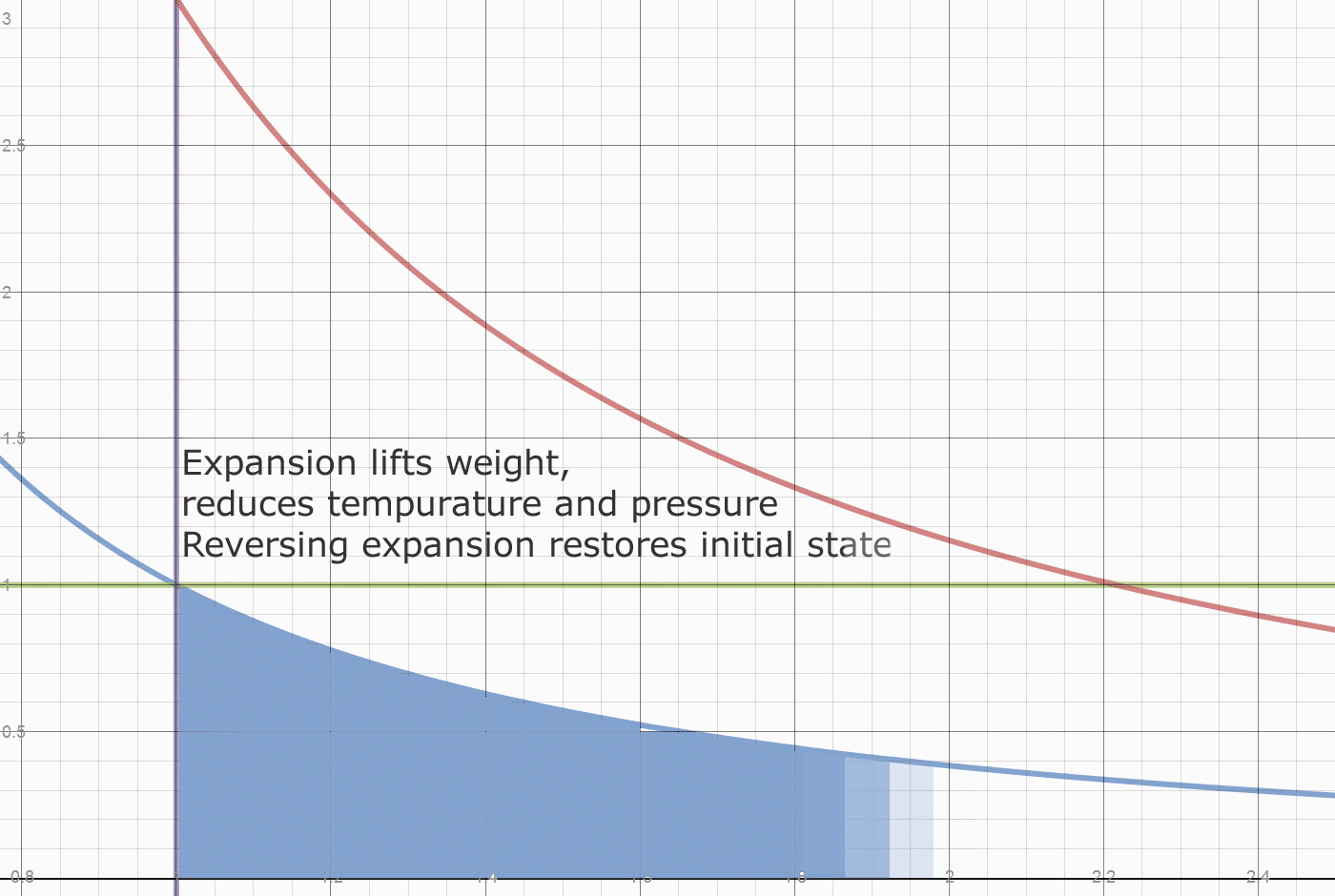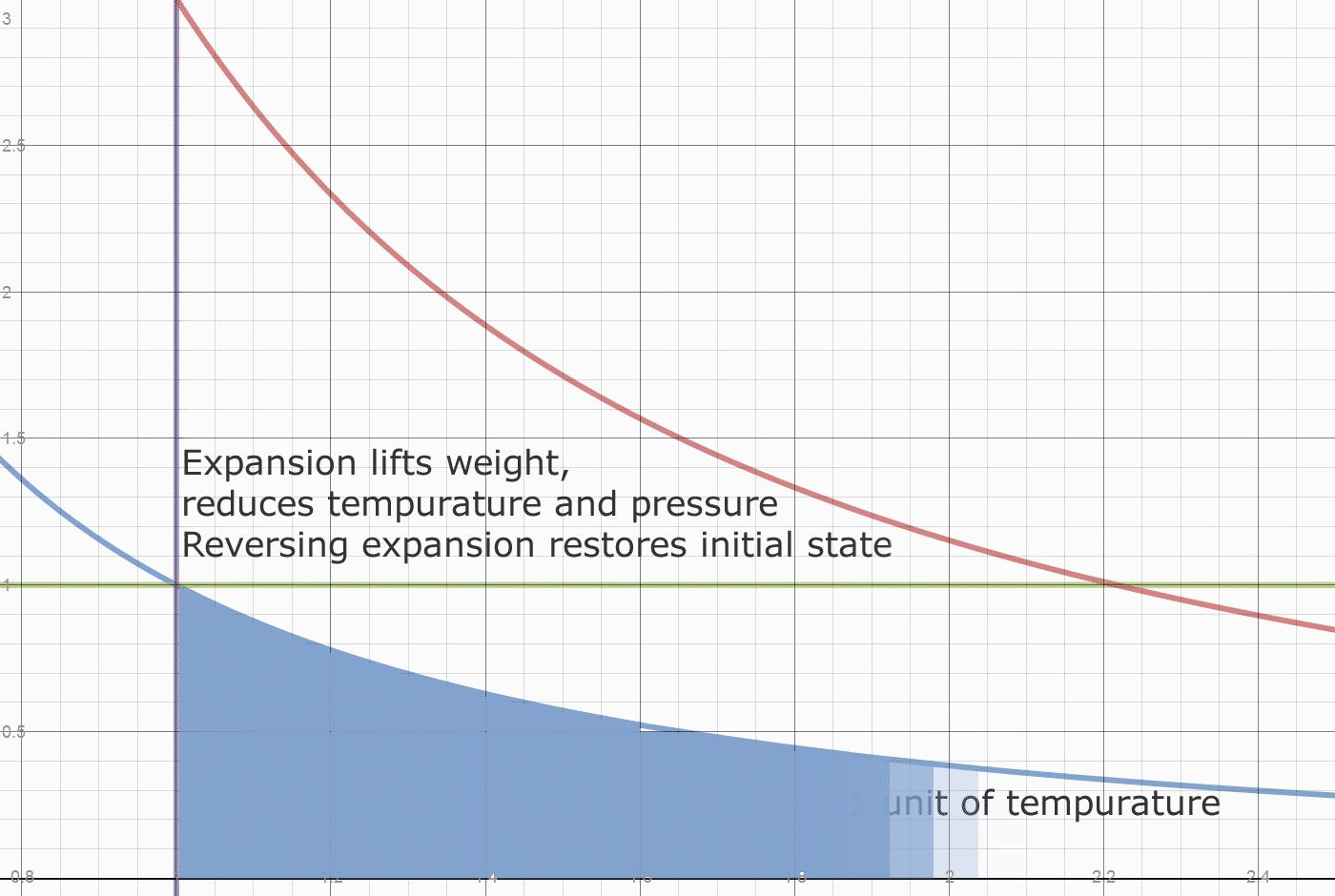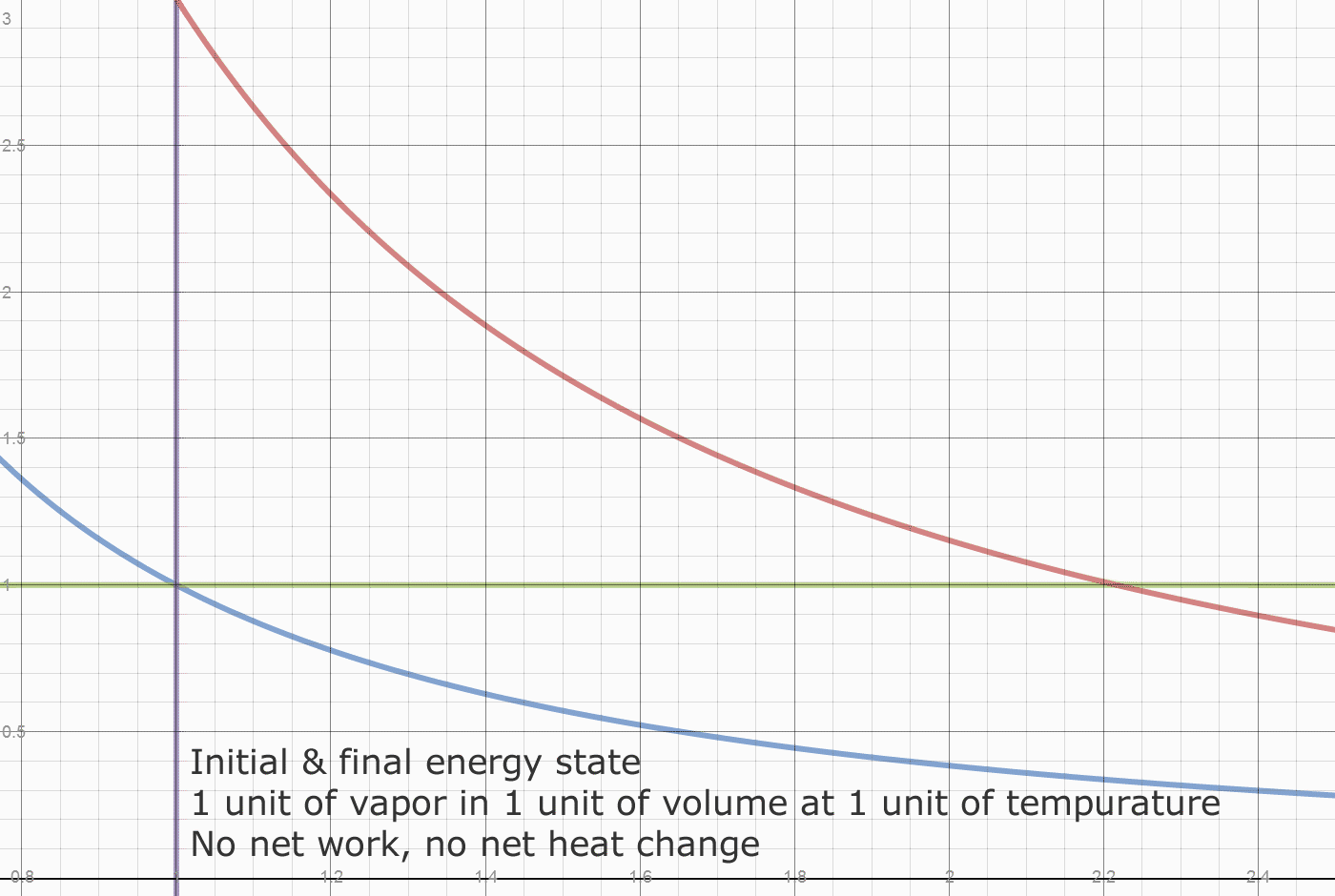
The next sequence shows how a two piston system can export 83% of the energy converted during expanding after a heat increase. While this is not yet near 100%, it far exceeds the "Carnot" (more accurately "Temperature Ratio") "Limit" of 66%. See the next page for approaching 100%.
|


