Corollaries and Limits.Up PreviousThere are several principles illustrated here. First, is an extension or generalization of the Third law. Corollary 1 to Third Law. No finite number of heat to work conversions, inside a pressure envelope, or constrained by a constant force, from expanding a vapor will return the vapor to its initial temperature. The proof relies on conversion asymmetry, any finite conversion leaves some heat. Because of the external constraining force, the expansion will stop before the initial temperature is again reached. (The alternative is a negative amount of exported work). Completely consuming added heat takes an infinite number of constrained expansions. Each expansion must reach a larger than initial volume. Each counter stroke beginning at the same temperature must still reach a larger than initial volume. Temperature can only drop during expansion. By "constrained" the initial conditions are that the pressure inside a constrained heat engine volume are in balance with the external force being applied in opposition to expansion. In other words, the behavior of thermodynamic transformations is always relative to its starting point of equilibrium, not to an absolute temperature or pressure. If instead we identify a delta, even a very small delta, then there is a finite number of conversions that will reach Initial Temperature + Delta. Corollary 2 to Third Law. Given any small increment Delta to temperature, a finite number of expansions in a finite number of volumes can return a closed system to within that Delta of its original temperature and volume. What does this mean?
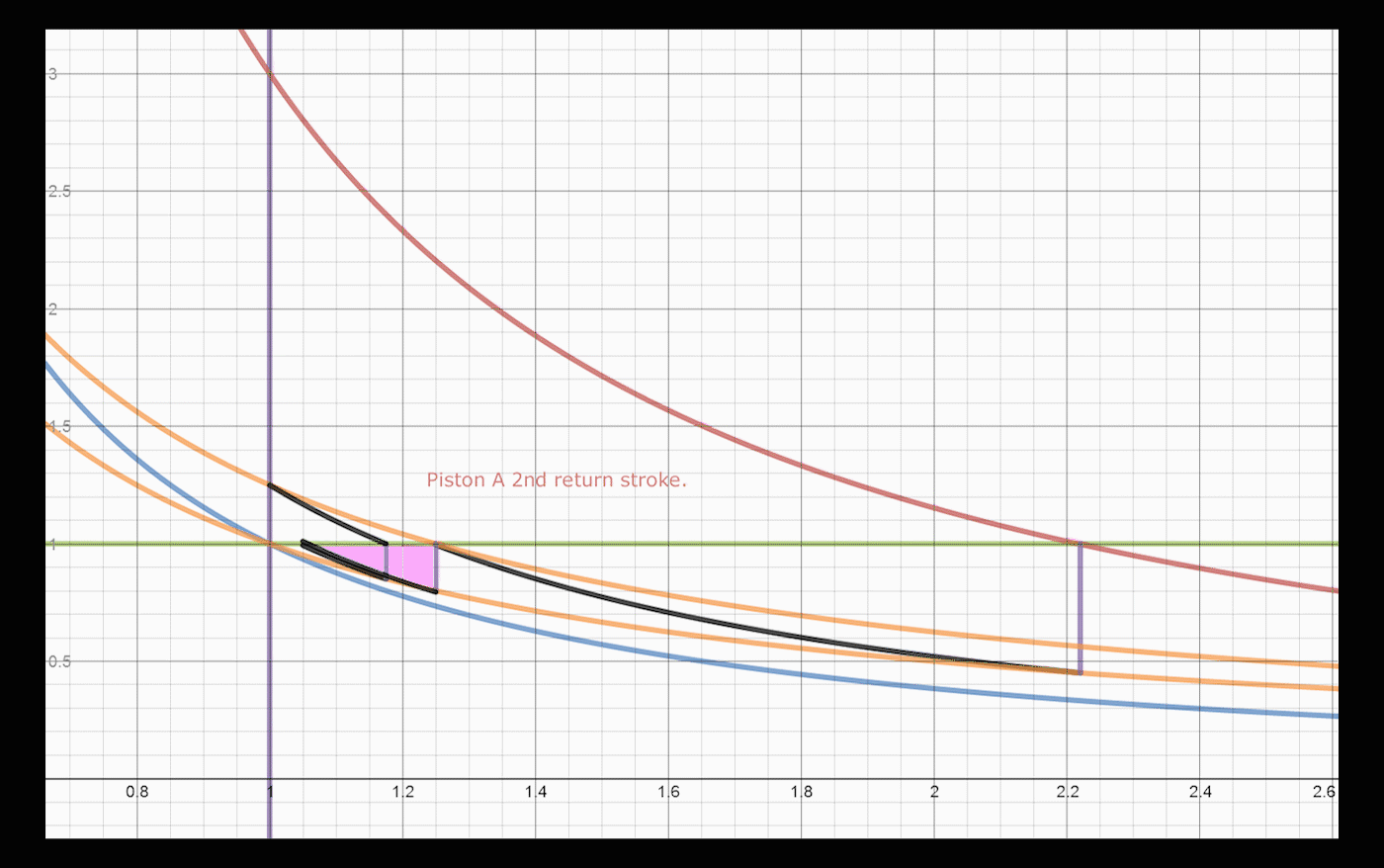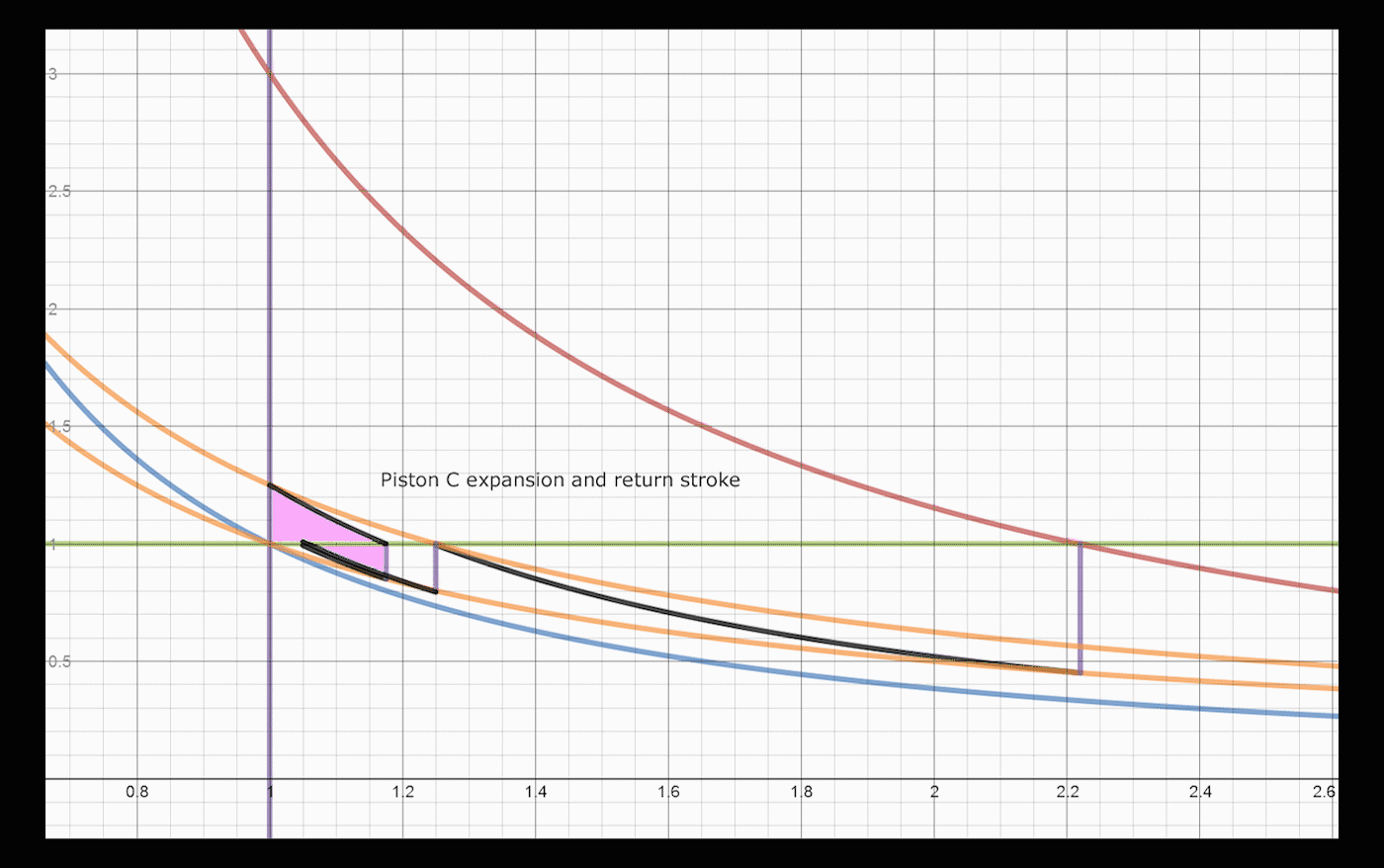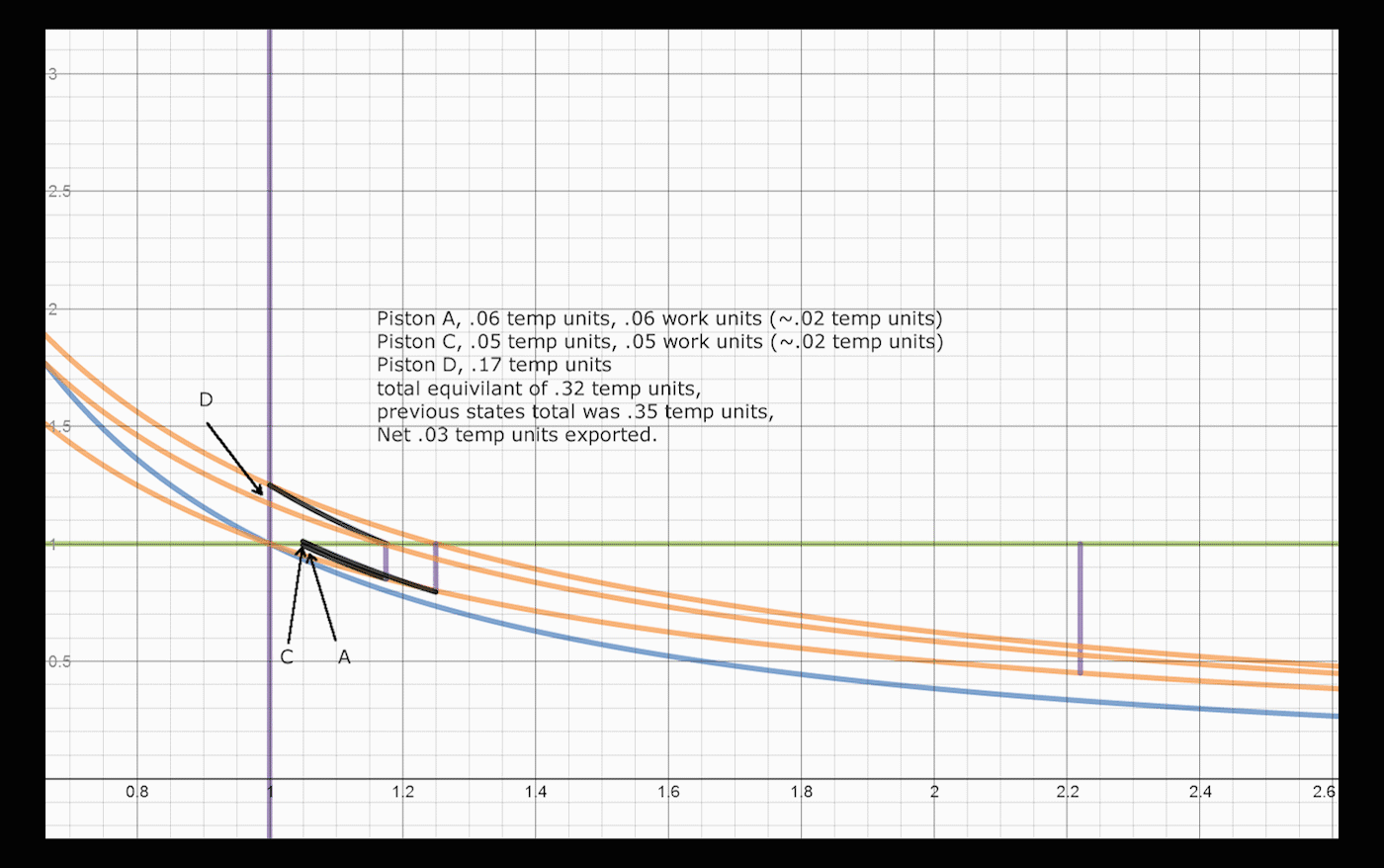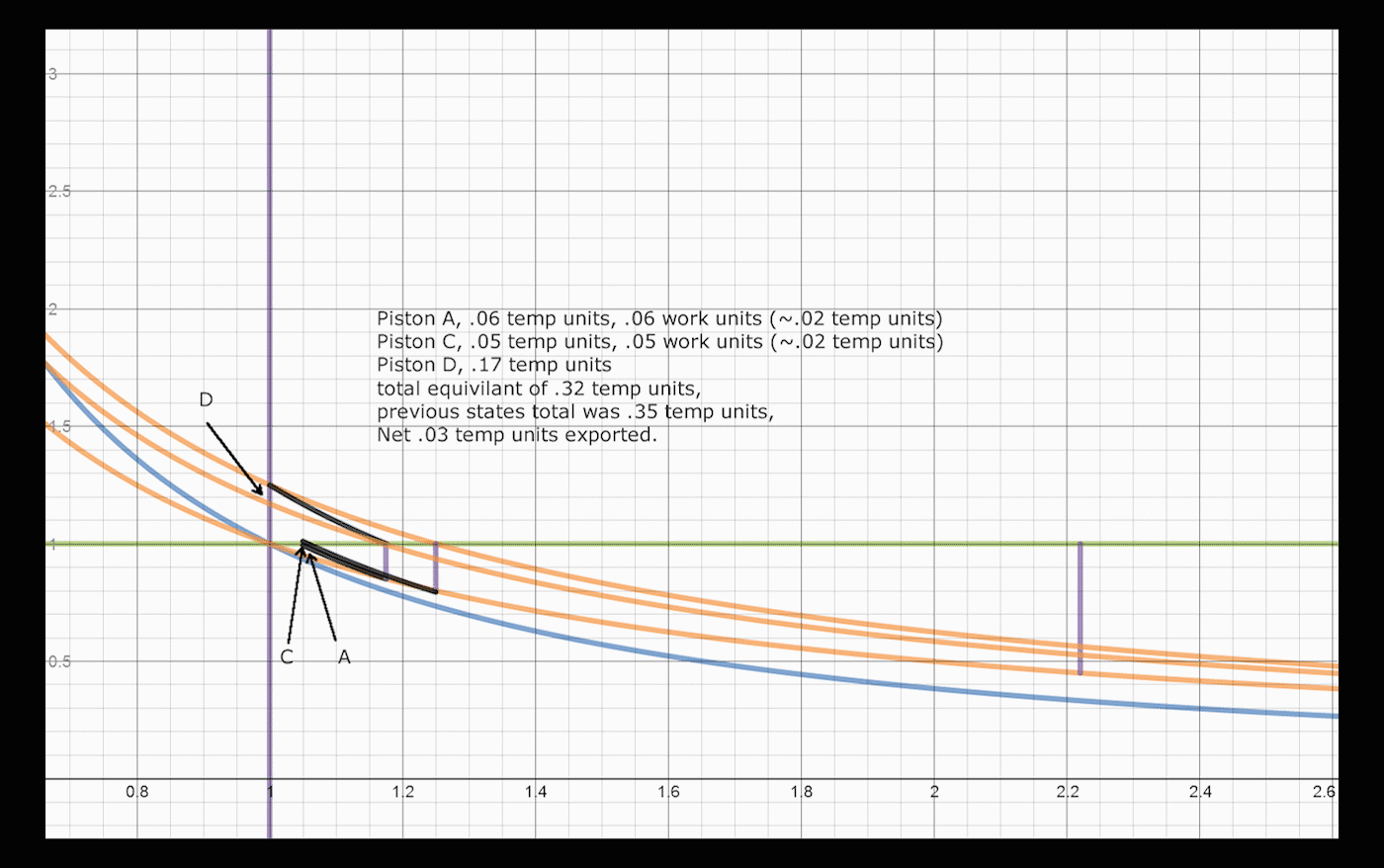
Below is a brief explanation of how one would add volumes and operations to the system, which would convert and export larger amounts of heat. The concept is recursive, one can always convert a significant amount of remaining energy by addition of a cylinder/piston pair. After a return stroke, Piston A has 1.25 heat units, and is at volume 1.25. This is the same state it was after the initial expansion, before heat was transferred to piston B, with a different amount of heat. Adding two cylinders allows the system to convert an extra 3% of the total energy, changing the total to 86% converted and exported, up from 83%. One can do a similar swap between Piston A and a third Piston C, which would allow:
. Once the Nth piston reaches within Delta/N of its original state, the remaining energy in the system can be iteratively converted, reusing the same pistons.
|