|
|
Simple steam recycling model demonstrates the 'Carnot Limit' fallacy.
Even a naive look at steam heat recycling does NOT support the "temperature ratio" limit.
- Below are "Carnot Limits" ranging from 32% to 46%, and computed efficiency from 56% to 98%. So clearly an exception to the "rule".
- Universal limits of the universe really should not have exceptions.
- Temperatures are not meaningfully comparable from inside to outside a heat engine.
- Temperatures are not proportional to energy between different substances.
- Temperature is not proportional to energy in the same substance between different solid, liquid or gas phases.
- Pressure is approximately proportional to energy.
- Pressure is meaningfully comparable between inside and outside of a heat engine.
Three cases of steam
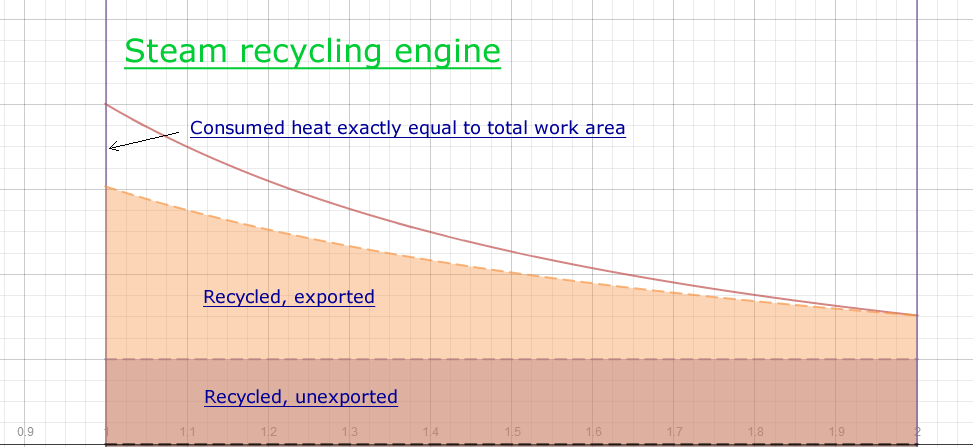
Case 1, Steam recycling low pressure
- pressure +3 atmospheres 4 total
- Temperature 132° C 405° K
- Clapeyron/Carnot Ratio ~77%
(expansion factor of 2)
- Temperature Ratio
(aka misnomer Carnot Limit)
1-300/447=32%
- Total work area ~(.23/.4)*4=2.3
- Work against pressure envelope, 1
- Efficiency (2.3-1)/2.3 = 56%
|
Graphs
|
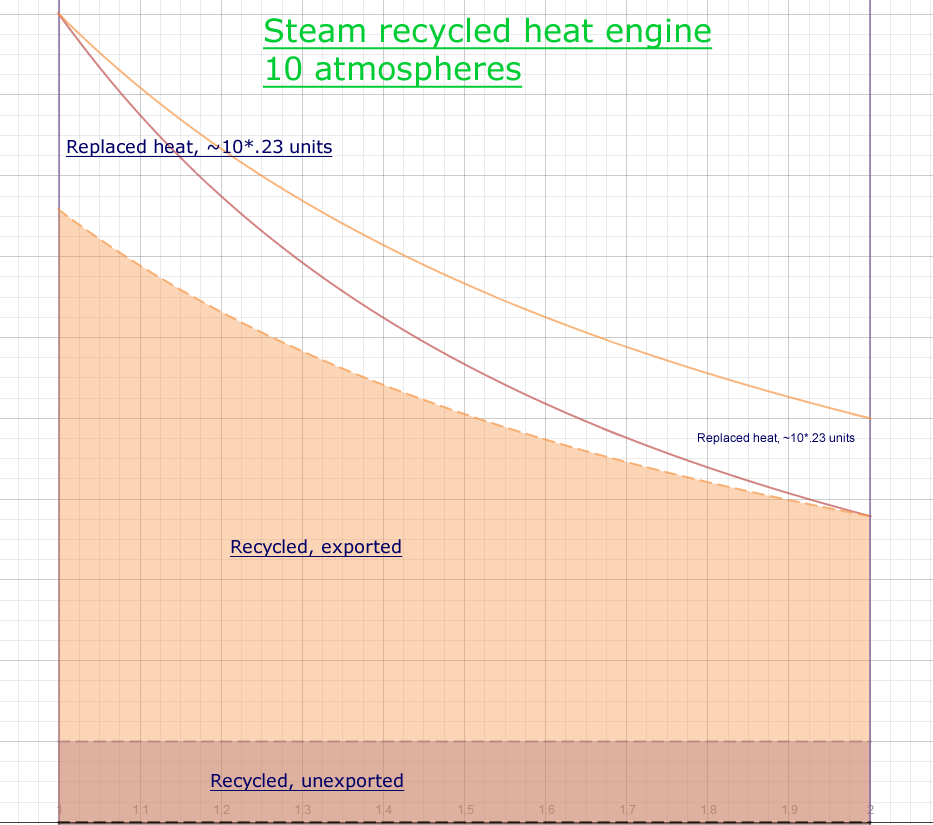
Case 2, Steam recycling medium pressure
- pressure +9 atmospheres, 10 total
- Temperature 174° C 447° K
- Clapeyron/Carnot Ratio ~77% (expansion factor of 2)
- Temperature Ratio
(aka misnomer Carnot Limit)
1-300/447=32%
- Total work area ~(.23/.4)*10=5.75
- Work against pressure envelope, 1
- Efficiency (5.75-1)/5.75= 83%
|
|
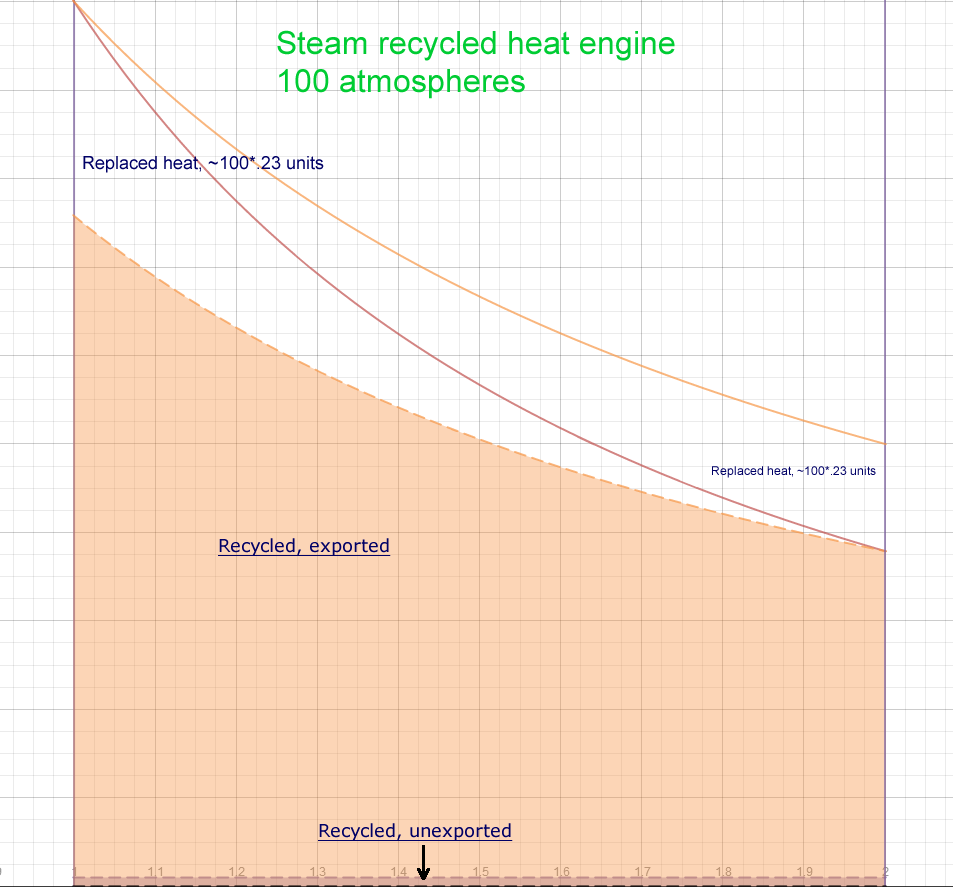
Case 3, Steam recycling High pressure
- Pressure +99 atmospheres 100 total
- Temperature 286° C, 559° K
- Clapeyron/Carnot Ratio ~77%
(expansion factor of 2)
- Temperature Ratio
(aka misnomer Carnot Limit)
1-300/559=46%
- Total work area ~(.23/.4)*100=57.5
- Work against pressure envelope, 1
- Efficiency (57.5-1)/57.5= 98%
|
|
|

