A Heat Engine is...
Well, one could argue heat engines don't exist. Heat can't move anything.
Heat engines are moved by pressure. They are fueled by heat. Pressure differences, the motive force, are created by taking vapor from a reservoir, constraining its volume, adding heat to create a pressure difference, then allowing expansion.
Notice we did not mention a cold reservoir or the temperature of the outside air?
Heat engines typically effect a fixed volume expansion. The output of any heat engine is the average pressure difference it creates, times the actual volume change it creates. This change gives the Work Ratio of an engine. The Work Ratio can be more or less than the Fuel Efficiency, as these are related, but not the same.
How much heat to add to raise the pressure? What if the vapor is cold or hot? Doesn't the energy needed to increase the pressure change? No. Equal energy increase (say, +300° of heat) yields equal pressure increase, no matter what the starting temperature. So your car runs the same when outside the temperature is 100 below zero or 100 above.
It does not matter what the temperature is outside, only the pressure. If, like most heat engines, it exhausts its vapor, it must exhaust vapor at slightly higher than ambient pressure. The outside can be a million degrees or near absolute zero. All that matters is pressure.
|
A Heat Engine Is NOT...
…Operated between two temperature reservoirs. No heat engine uses either heat reservoirs or cold reservoirs. It’s an abstract idea that is, in fact, very misleading.
No existing heat engines benefit or produce work from a cold reservoir. They all take a vapor of ANY temperature, enclose and create a pressure difference by adding heat energy. If the vapor temperature was 100 below zero or 400 above zero, the engine would work exactly the same.
Strictly speaking, a "cold source" is not possible. We can make heat sources, which convert electrical, mechanical, or chemical energy into heat. Nothing absorbs heat and maintains the same temperature (some reactions absorb limited heat, but none create a regulated temperature). If one did have a "cold source" it could theoretically be made to create pressure differences, and therefore a motive force for a heat driven engine, by reducing pressure instead of increasing it. Purely imaginary, at least till someone cleverer comes along. Even so, the engine would be fueled by heat, the vapor from the hotter-higher pressure side (possibly the envelope) expanding into a cooled, hence lower pressure, volume. No free lunch. If you did have a cold source, you still need a heat source.
Heat engines cannot tell what the outside temperature is. They can only tell what the outside pressure is.
So, unbelievers, where do you add a reservoir of liquid nitrogen to improve the performance, efficiency, or work output of a 4 cycle engine?, a steam turbine? A fossil fuel turbine? Jet Engine? Can't be done.
The "reservoir" model is the same as the water wheel model, that heat is an indestructible constant volume fluid, the model which predated the concepts of conservation of energy and the laws of thermodynamics.
It’s a common misconception that the outside air’s temperature would limit a heat engine’s range. This isn’t so - in fact, a viable engine cycle (if not optimal) engine cycle that exhausts air colder than the air it took in could be built. How?
- Do isothermal compression. Then...
- Add just a little less heat than the isothermal step threw away...
- Allow it to expand, forcing expansion to continue to the original volume (such as via momentum)...
- The final temperature will be below the starting temperature.
- It will make a positive work cycle, kind of distorted figure 8.
- Exhaust at well below the starting ambient temperature.
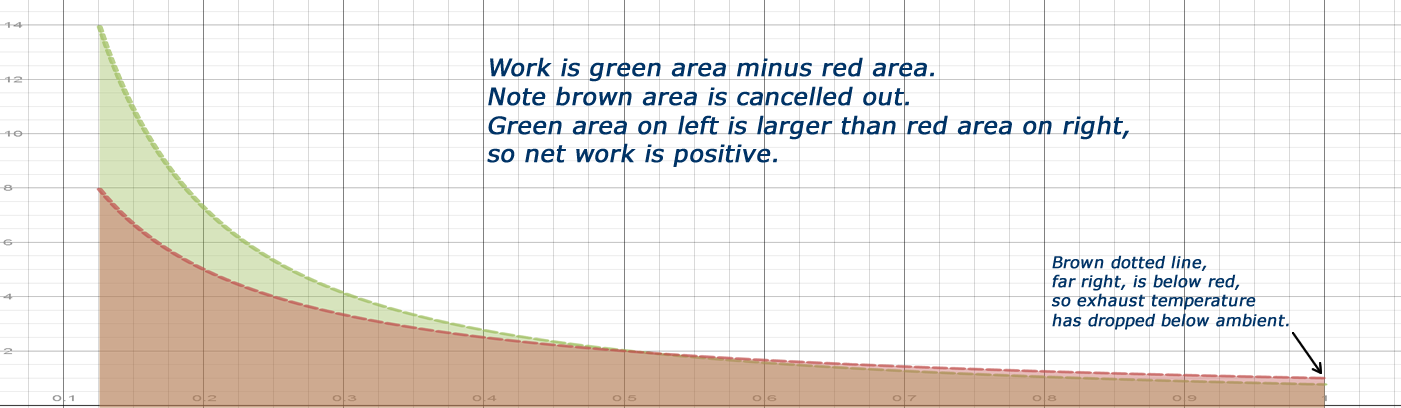
Typical 4 cycle engines heat the air during compression. There is no theoretical limit, and a very high practical limit to how much heating can be done. Neither the upper or lower bounds of vapor temperature have anything to do with ambient temperature outside, nor are they limited by the temperature rise from fuel combustion. Even in the abstract, no engines operate between fixed temperatures.
|

