|
Proof is that Diffusion of heat is Waste.
This could be considered a corollary to Carnot's assertion about diffusion.
Insulated construction is the only known construction method for eliminating diffusion. May seem trivial, but as almost all Heat Engines 170 years later are still metal, apparently not obvious to everyone.
|
Theorem K2: Ideal Construction is Impermeable to Heat
Sadi Carnot did not describe construction of his cylinder, possibly he thought it was obvious. In any case, Lord Kelvin's spells it out. An Ideal construction is impermeable (insulated).
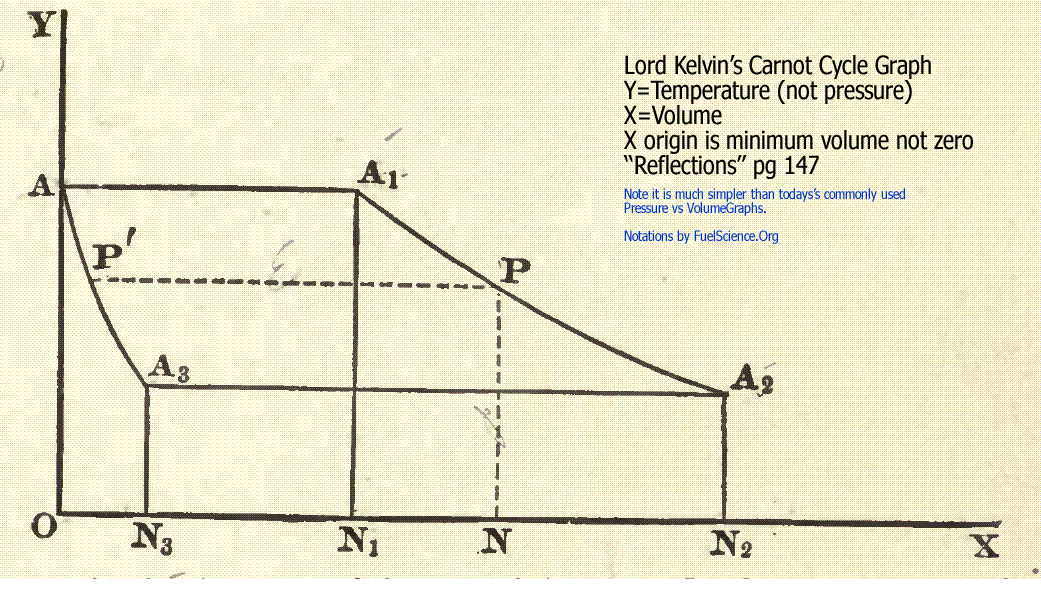
"Reflections" pg 141 (steam engines) by Lord Kelvin
paragraph 15. Let CDFE be a cylinder, of which the
curved surface is perfectly impermeable to heat,
with a piston also impermeable to heat, fitted in it ;
while the fixed bottom CD, itself with no capacity
for heat, is possessed of perfect conducting power.
Let K be an impermeable stand, such that when
the cylinder is placed upon it the contents below
the piston can neither gain nor lose heat.
|
pg 152 (Air engines) by Lord Kelvin
paragraph 22. In the ideal air-engine imagined by Carnot
four operations performed upon a mass of air or
gas enclosed in a closed vessel of variable volume
constitute a complete cycle, at the end of which
the medium is left in its primitive physical condition;
the construction being the same as that which
was described above for the steam-engine, a body
A, permanently retained at the temperature 8, and
B at the temperature T\ an impermeable stand K\
and a cylinder and piston, which in this case contains
a mass of air at the temperature S, instead
of water in the liquid state, at the beginning and
end of a cycle of operations. The four successive
operations are conducted in the following manner :
|