|
|
Assertions and theorems inconsistent with current physics models of heat, gases, movement and energy.
Some conclusions and some measurement methodologies in "Reflections" were flawed.
Contradictions were noted by both Carnot and Kelvin. Nevertheless, the truly fundamental conclusions were all valid.
Heat & Motion energy are conserved. Heat has a fixed potential for produhalcing motion, independent of the engine used.
Heat is converted to motion only via Volume Change. Energy is proportional to relative, not absolute, volume change.
Commentary
Air increases in temperature according to the amount of work done. The methodology of these measurements is flawed, because it was done on a variety of gases, but all at the same pressure. The work done on the gas is Volume change * average Pressure.
|
Disproved Theorems
Disproved Theorem Z1: Air temperature increases one degree C for a fixed relative compression.
"Reflections", page 73, Sadi Carnot Quoted by Editor
Chapter Motive Power of Heat
Atmospheric air should rise one degree Centigrade
when by sudden compression it experiences
a reduction of volume of 1/116. |
|
It's hard to classify this one, because the measurements were correct, but the conclusion incorrect.
The "Modern" way of calculating this can be found in Wikipedia (until they read this). Heat up a gas in an expandable chamber and a fixed sized chamber the same amount, then let the fixed sized chamber expand. Then take the difference in temperature This measures the amount of heat drawn from the gas, which is a function of its specific heat. The amount of work is primarily a function of its initial pressure, but a second order difference of Volume-Bk,where Bk = Gamma-1, also occurs. So, the "Modern" method will yield a different answer than the "old" method. This method mixes properties of the gas with properties of the work, both easily miss-measured. Doing this measurement with helium will yield a larger temperature shift than air.
The Old method is to measure the amount of heat applied, a much more difficult experiment. It precisely measures the quantity of heat given for a fixed volume and a "fixed" pressure. In this case, it will always take exactly the same increment of heat to reach the larger volume. Doing this measurement with helium will yield the same answer as air.
The larger volume is determined by the ideal gas law, which is the same for all gases, and does not depend on the amount of heat stored in the gas. In either method, the work done for constant pressure is always Pressure times Volume Change. This method gives an accurate and constant measurement of the heat difference. However, by far the simplest way of creating "constant" pressure is to simply produce a free piston with one side at atmospheric pressure. So experiments that had great precision would yield different results literally as the weather changed. Huge differences would occur at different altitudes.
Neither method recognizes the extra heat is not a property of the gas, but of the volume change and pressure.
Oversight? No, its a very subtle measurement. It might seem an insignificant amount of work. Say the test cylinder had 10 square inches of stopper (piston). The laboratory technician could easily move the piston up and down several inches, till air pressure difference made it difficult. Increasing the temperature from 30°C to 31°C made a volume difference of 1/300 of its total volume. Almost imperceptible. The force on each side of the stopper is 1 atmosphere, or 15 psi or 150 pounds total. Lifting a 150 pound person even a slight amount takes a lot of work. The forces being in balance make the amount of work being done difficult to perceive.
So if all gases have the same "extra heat" to fill extra volume, it suggests its the volume itself which takes the extra heat. Not ignorant 1800's scientists, precise measurements with repeatable results. But the conclusion led to contradictions. Double or halve the fixed volume of a gas, then measure its specific heat, its always the same at every fixed volume. So tiny volume changes take large amounts of heat, and Large volumes take no heat. Sadi Carnot and Lord Kelvin both refer to "contradictory information", and the Specific Heat of Volume makes for more striking and obvious contradictions than Materiality of Heat.
|
Disproved Theorem Z2: The specific heat of volume is the same for all gases.
"Reflections", page 76, Sadi Carnot
Chapter Motive Power of Heat
The difference between specific heat under constant
pressure and specific heat under constant
volume is the same for all gases.
It is a very easy matter now for us to prepare a
table of the specific heat of gases under constant
volume, from the knowledge of their specific heats
under constant pressure. Here is the table :
TABLE OF THE SPECIFIC HEAT OF GASES.
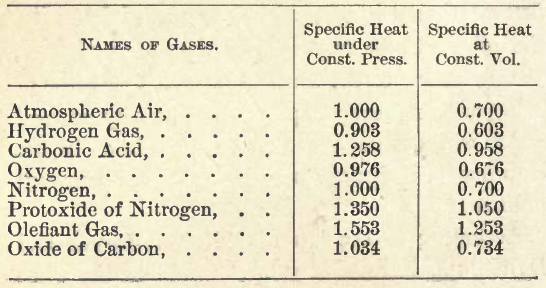 The first column is the result of the direct
experiments of MM. Delaroche and Berard on the
specific heat of the gas under atmospheric pressure,
and the second column is composed of the numbers
of the first diminished by 0.300.
The first column is the result of the direct
experiments of MM. Delaroche and Berard on the
specific heat of the gas under atmospheric pressure,
and the second column is composed of the numbers
of the first diminished by 0.300. |
|
First comment, this theorem is the exact opposite of the usual interpretation of the "Carnot Work Ratio". The usual interpretation is higher temperatures yield higher efficiency, (which is wrong.)
The ideal gas law says that volume or pressure increase proportional to absolute temperature so at 100 degrees kelvin (cold), 300 degrees (room temperature, or 1000 degrees (hot enough to melt several types of metals), a 1 degree increase increases volume by 1/100, 1/300, and 1/1000. Which if one multiplies times the volume, 1 degree yields 1 additional unit of volume at a given pressure. So every degree produces exactly the same total (not relative) volume increase. "Efficiency" neither increases nor decreases with temperature
|
Disproved Theorem Z3: The Motive Power of Heat reduces with temperature
"Reflections", page 92, Sadi Carnot
Chapter Motive Power of Heat
The quantity of heat due to the change of volume
of a gas is greater as the temperature is higher.
page 96, Sadi Carnot
Chapter Motive Power of Heat
The fall of caloric produces more motive power at
inferior than at superior temperatures.
Its unclear where these conclusions came from or were based upon.
It is noteworthy the conclusions would appear to be disproved by the above table of "Specific heats"
|
|
Disproof. The simplest disproof is a counter example.
Consider an ideal cylinder and piston, of great length, in a vacuum. It starts with a vapor at some pressure and temperature
The volume will expand until all it's heat has been converted to work. There is no cold body, a vacuum is temperature-less. vacuums cannot hold heat, nor are they cold. They are near perfect insulators, except for energy in other forms than motion.
Law of Conservation of Energy also requires Heat to be Consumed to produce Motion in a heat engine.
|
Disproved Theorem Z4: The Motive Power of Heat requires Cold.
"Reflections", page 46, Sadi Carnot
Chapter Motive Power of Heat
The production of motive power is then due in
steam-engines not to an actual consumption of
caloric, but to its transportation from a warm
body to a cold body, that is, to its re-establishment
of equilibrium an equilibrium considered as destroyed
by any cause whatever, by chemical action
such as combustion, or by any other. We shall
see shortly that this principle is applicable to
any machine set in motion by heat.
According to this principle, the production of
heat alone is not sufficient to give birth to the
impelling power: it is necessary that there should
also be cold; without it, the heat would be useless. |
|
Disproof. The simplest disproof is a counter example.
Among the oldest machines are the Sail and the Windmill. Both of these work by the same manner as all vapor expansion machines. They convert Pressure * Volume to energy, where volume is the volume swept by wind.
When wind strikes a sail or windmill blade, they are moving away from the wind, usually on a diagonal. The air molecules bounce off the surface moving away, and their speed is reduced by the momentum they impart to the machine. Slower molecules is cooler molecules.
While no one has figured out how to do this without weather cooperating, it is clearly a thermodynamic process which is operating in constant temperature It is powered by the speed (heat) of the air molecules. Those molecules are cooled below their original temperature, because they gave off energy.
|
Disproved Theorem Z5: It is not possible to convert ambient heat to work.
Lord Kelvin expressed the second law (of thermodynamics) as "It is impossible, by means of inanimate material agency, to derive mechanical effect from any portion of matter by cooling it below the temperature of the coldest of the surrounding objects."
Credited to: Thomson, W. (March 1851). "On the Dynamical Theory of Heat, with numerical results deduced from Mr Joule’s equivalent of a Thermal Unit, and M. Regnault’s Observations on Steam". Transactions of the Royal Society of Edinburgh XX (part II): 261-268; 289-298. Also published in Thomson, W. (December 1852). "On the Dynamical Theory of Heat, with numerical results deduced from Mr Joule’s equivalent of a Thermal Unit, and M. Regnault’s Observations on Steam". Philos. Mag. 4 IV (22): 8-21. Retrieved 25 June 2012, by Wikipedia. Citation not verified by FuelScience.org.
|
|

